Expert Glaucoma Treatment Fort Worth

If you or a loved one are in need of specialized care for glaucoma in fort worth, it’s crucial to find a skilled and experienced medical doctor who can provide the highest level of treatment and support.
Dr Brian Flowers is a leading glaucoma doctor in fort worth with over 25 years of experience in glaucoma diagnosis, glaucoma management and glaucoma surgical interventions.
Brief background of Brian Flowers, M.D.
Dr. Flowers joined Ophthalmology Associates (OA) in 1998. Over the years Dr. Flowers has remained at the forefront of Glaucoma diagnosis and treatment and is responsible for bringing much of the latest in technology to our area.
These firsts include measuring corneal thickness to accurately measure intraocular pressure, the first optic nerve head analyzers, endoscopic laser surgery, non-scarring selective laser trabeculoplasty, and most recently, non-penetrating glaucoma surgery (canaloplasty), and minimally invasive glaucoma surgery. These represent nearly every advancement in glaucoma care that has occurred over the past 20 years. In 2021 he was awarded the 12th annual Surgery Day Lecture at the American Glaucoma Society national meeting.
Why Choose Brian Flowers, M.D?
- Award-winning Specialist
- High Treatment Success Rate
- 25+ Years of Experience
Fort Worth Glaucoma Treatment
What is Glaucoma?
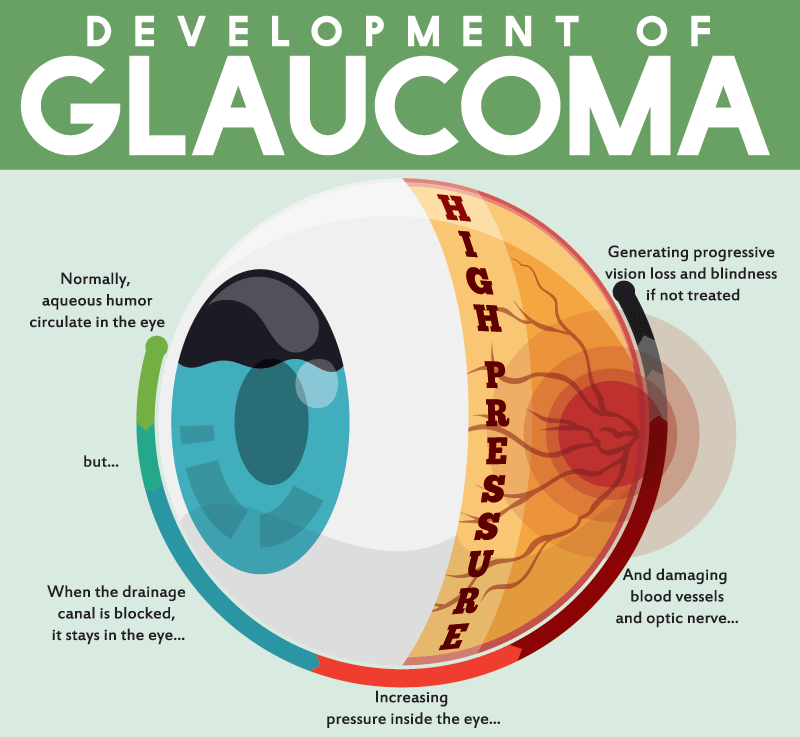
Glaucoma is a form of damage to the optic nerve in the back of the eye. The optic nerve connects your eye to your brain and transmits the information we need to be able to see. The damage can be caused by excessive pressure inside your eye (intraocular pressure or IOP) and other factors.
What is the Consequence of Glaucoma?
With optic nerve damage, you may begin to lose your peripheral vision. Your peripheral vision is that vision that is outside of your central gaze. Over time, if glaucoma damage is severe, you may begin to lose your central vision as well.
What are the Different Types of Glaucoma?
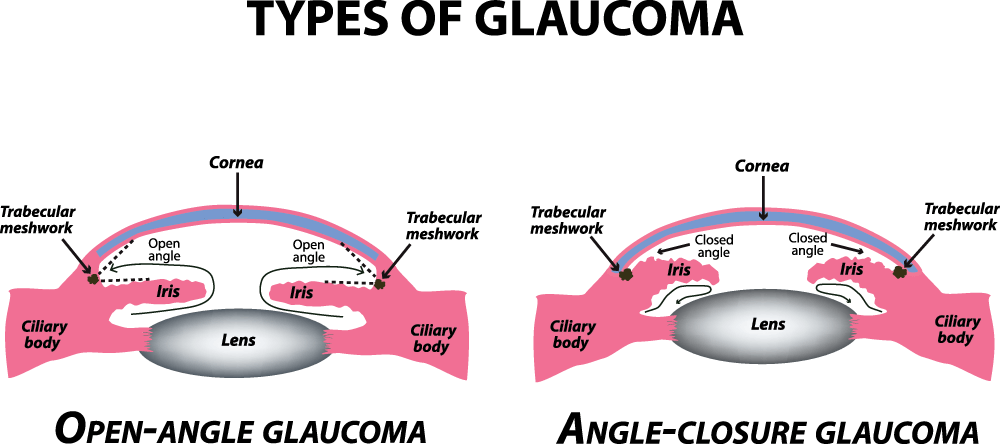
The primary forms of glaucoma are called open-angle and closed-angle glaucoma.
What is the Angle of the Eye?
The angle refers to space in the very front part of your eye, between the cornea and the iris. It is where the fluid the eye produces exits the eye.
What is Open-Angle Glaucoma?
Your eye produces an internal fluid called aqueous humor. Aqueous humor drainage takes place in a structure called the trabecular meshwork, located in the angle of the eye. In open-angle glaucoma, there is a malfunction to the trabecular meshwork (black box in the picture). We do not know precisely what causes this malfunction. We do know that there are known risk factors, such as age, a family history of glaucoma, history of taking steroid medication or prior trauma. As a consequence of the malfunction, aqueous fluid doesn’t drain quickly enough, causing the IOP to become elevated, leading to optic nerve damage.
What is Closed-Angle Glaucoma?
As seen in this picture the aqueous fluid has a difficult time passing between the iris and the lens. The pressure of the fluid pushes the iris forward leading to a narrowing of the angle. This, in turn, can lead to optic nerve damage. IOP can be elevated in this form of glaucoma, but not always.
How Can my Glaucoma Specialist Know if I Have Open Vs. Closed-Angle Glaucoma?
Your glaucoma specialist uses a specialized contact lens called a gonioprism to evaluate the angle. This is a painless assessment done during the course of your eye exam.
I Have High Blood Pressure. Is This Causing My High IOP?
No. There is no clear association between elevated blood pressure and IOP. Blood pressure relates to the pressure of blood in your blood vessels. IOP elevation usually relates to a malfunction in the drainage system of the eye, preventing normal aqueous fluid drainage.
My Glaucoma Specialist Told Me That I Am A “Glaucoma Suspect.” What Does This Mean?
This means that your eyes have some risk factors for glaucoma, but there is no clear damage to the optic nerve. Based on the risk factors present, your glaucoma specialist can give you some perspective on the likelihood of you developing glaucoma in the future. We typically monitor glaucoma suspects once or twice a year and recommend treatment if it is felt that the risk of developing glaucoma is increasing.
My Physician Told me That I Have Glaucoma. Am I Going to go Blind?
Fortunately, with proper treatment and follow-up, blindness from glaucoma can normally be prevented.
Is There a Cure for Glaucoma?
At present, there is no cure for glaucoma, but proper treatment usually prevents vision loss. Read more about glaucoma treatment options.
The Glaucoma Consultation
What can I expect at my glaucoma consultation visit?
A glaucoma evaluation requires multiple pieces of information. Your glaucoma team will check your vision and IOP and other various features of the eye. Imaging in the form of a very brief picture test often aids your glaucoma specialist in evaluating your optic nerve. Your glaucoma specialist will sit down with you, evaluate your eye and IOP and discuss the findings.
I’ve had my IOP checked at my regular eye doctor’s office. Do I need my IOP checked again?
It is important that your glaucoma specialist do an independent evaluation of your eye and your IOP.
What is the purpose of the visual field test?
The visual field test is a simple office test that evaluates your peripheral vision. This aids your glaucoma specialist in assessing the severity of your glaucoma.
I have great peripheral vision, why do I need to take this test?
No matter how great you think your peripheral vision is, you may still have glaucoma damage which can only be detected by visual field testing.
